Draw the ammonium salt formed in each reaction – In the realm of chemistry, ammonium salts play a pivotal role in a myriad of reactions. This comprehensive guide delves into the intricate mechanisms underlying their formation, explores their unique properties, and unveils the analytical techniques employed to identify and quantify these versatile compounds.
As we embark on this journey, we will uncover the fascinating world of ammonium salts, their applications, and the safety considerations associated with their handling.
Ammonium salts, characterized by their characteristic cation NH4+, arise from the neutralization reactions between acids and bases. These reactions, governed by the principles of acid-base chemistry, yield ammonium salts that possess distinct properties from their parent acids and bases. Understanding the formation and properties of ammonium salts is essential for comprehending their diverse applications in various fields, including fertilizers, pharmaceuticals, and industrial processes.
Reaction Mechanisms
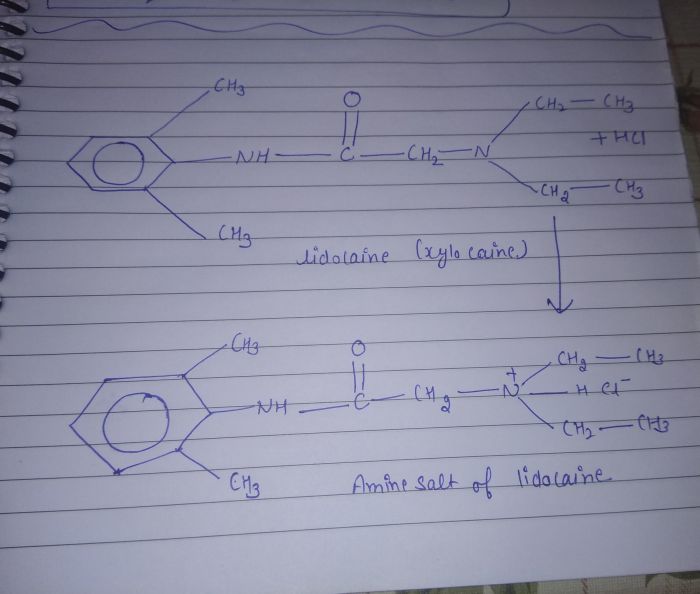
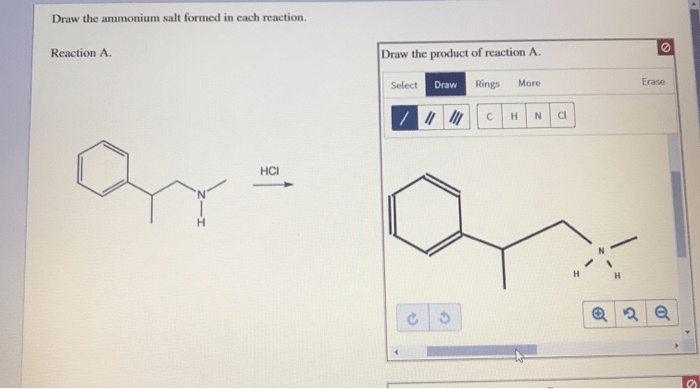
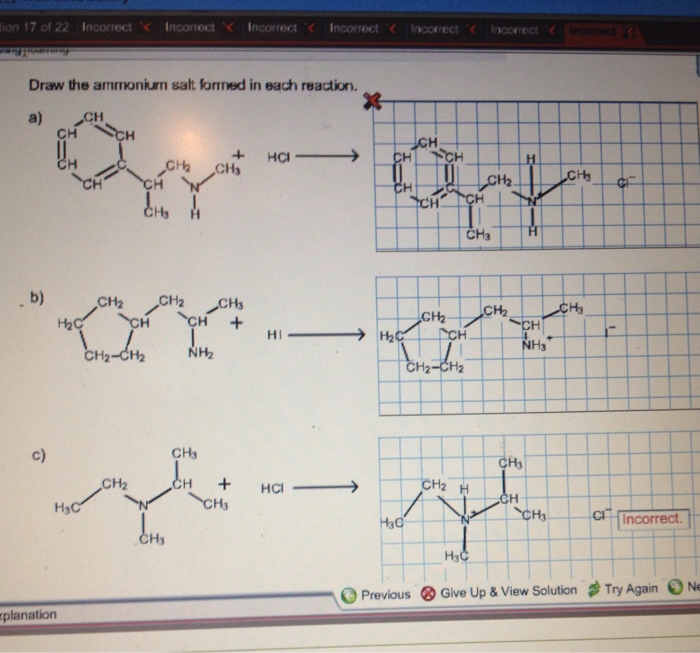
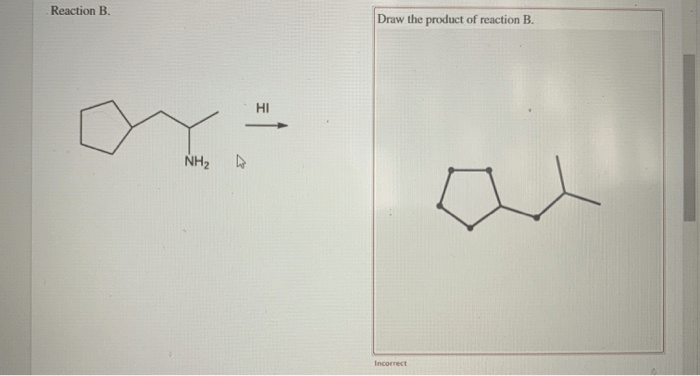
Ammonium salts are formed through acid-base reactions between an acid and a base. In these reactions, the acid donates a proton (H +) to the base, forming an ammonium ion (NH 4+) and an anion (the conjugate base of the acid).
For example, when ammonia (NH 3) reacts with hydrochloric acid (HCl), the following reaction occurs:
NH3+ HCl → NH 4Cl
In this reaction, ammonia acts as the base and accepts a proton from hydrochloric acid, forming ammonium chloride (NH 4Cl) as the ammonium salt.
Role of Acid-Base Reactions in Ammonium Salt Formation, Draw the ammonium salt formed in each reaction
Acid-base reactions play a crucial role in the formation of ammonium salts. The strength of the acid and base involved determines the extent to which the reaction proceeds and the stability of the resulting ammonium salt.
Strong acids, such as hydrochloric acid, readily donate protons and form stable ammonium salts with weak bases, such as ammonia. Conversely, weak acids, such as acetic acid, do not readily donate protons and form less stable ammonium salts with strong bases.
Properties of Ammonium Salts: Draw The Ammonium Salt Formed In Each Reaction
Ammonium salts are typically white or colorless crystalline solids that are soluble in water. They have a characteristic pungent odor and a bitter taste.
Physical and Chemical Properties of Ammonium Salts
- Physical Properties:Ammonium salts are typically solids at room temperature and have high melting and boiling points. They are also hygroscopic, meaning they absorb moisture from the air.
- Chemical Properties:Ammonium salts are generally stable compounds, but they can decompose upon heating or exposure to strong acids or bases. They undergo reactions such as hydrolysis, thermal decomposition, and precipitation.
Differences from Parent Acids and Bases
Ammonium salts have different properties compared to their parent acids and bases. They are typically less acidic or basic than their parent acids and bases due to the presence of the ammonium ion, which has a neutralizing effect.
Applications of Ammonium Salts
- Fertilizers:Ammonium salts, such as ammonium nitrate (NH 4NO 3) and ammonium sulfate ((NH 4) 2SO 4), are widely used as nitrogen fertilizers in agriculture.
- Explosives:Ammonium nitrate is also used in the production of explosives, such as dynamite.
- Food Additives:Ammonium salts, such as ammonium bicarbonate (NH 4HCO 3), are used as leavening agents in baking.
- Pharmaceuticals:Ammonium salts, such as ammonium chloride (NH 4Cl), are used in cough syrups and expectorants.
Analytical Techniques
Various analytical techniques are used to identify and quantify ammonium salts in different samples.
Principles of Analytical Techniques
- Ion Chromatography:This technique separates ions based on their charge and size, allowing for the identification and quantification of ammonium ions in a sample.
- Spectrophotometry:This technique measures the absorbance of light by a sample at specific wavelengths, allowing for the determination of the concentration of ammonium ions.
- Titration:This technique involves adding a known concentration of a reagent to a sample until a reaction endpoint is reached, allowing for the determination of the concentration of ammonium ions.
Applications in Practice
- Environmental Monitoring:Ammonium salts are analyzed in water and soil samples to assess the levels of nitrogen pollution.
- Food Analysis:Ammonium salts are analyzed in food products to ensure compliance with safety regulations.
- Clinical Chemistry:Ammonium salts are analyzed in blood and urine samples to diagnose metabolic disorders.
Safety Considerations
Ammonium salts can pose certain hazards when handled or stored improperly.
Hazards Associated with Ammonium Salts
- Inhalation:Inhalation of ammonium salts can cause irritation to the respiratory tract.
- Skin Contact:Contact with ammonium salts can cause skin irritation or burns.
- Eye Contact:Contact with ammonium salts can cause eye irritation or burns.
- Ingestion:Ingestion of ammonium salts can cause gastrointestinal distress.
Proper Safety Precautions
- Handling:Ammonium salts should be handled in well-ventilated areas and with appropriate personal protective equipment (PPE), such as gloves, safety glasses, and a respirator.
- Storage:Ammonium salts should be stored in a cool, dry place away from incompatible materials, such as acids and bases.
- Disposal:Ammonium salts should be disposed of according to local regulations and guidelines.
Environmental Impacts of Ammonium Salts
Ammonium salts can have adverse effects on the environment if released into water bodies or soil.
- Water Pollution:Ammonium salts can contribute to eutrophication, a process that leads to the depletion of oxygen in water bodies, harming aquatic life.
- Soil Pollution:Ammonium salts can accumulate in soil, leading to acidification and reduced soil fertility.
Quick FAQs
What are the key factors influencing the formation of ammonium salts?
The formation of ammonium salts is primarily governed by the strength of the acid and base involved in the reaction. Stronger acids and bases tend to produce ammonium salts more readily.
How can we distinguish ammonium salts from other types of salts?
Ammonium salts can be distinguished from other salts by their characteristic properties, such as their solubility in water, their ability to form complexes with metal ions, and their thermal stability.
What are the potential hazards associated with handling ammonium salts?
Ammonium salts can be toxic if ingested or inhaled, and they can also cause skin and eye irritation. Proper safety precautions, including the use of personal protective equipment and adequate ventilation, should be taken when working with these compounds.