In the realm of chemistry, the net ionic equation for sodium sulfate and barium chloride holds a significant position. It provides a simplified representation of the chemical reaction between these two compounds, revealing the essential ions involved in the process.
Understanding this net ionic equation not only enhances our comprehension of the reaction but also offers valuable insights into various applications and limitations within the field.
Delving deeper into the topic, we will explore the concept of net ionic equations, identify the spectator ions in the reaction between sodium sulfate and barium chloride, and derive the net ionic equation. Furthermore, we will shed light on the practical applications of net ionic equations in diverse areas of chemistry, such as qualitative analysis and solubility predictions.
Net Ionic Equations: Net Ionic Equation For Sodium Sulfate And Barium Chloride
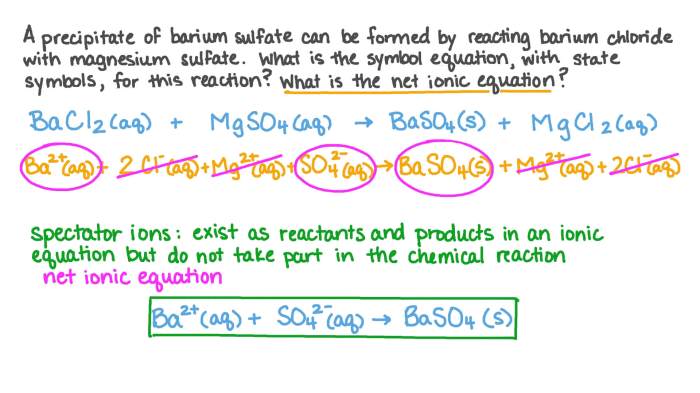
A net ionic equation is a chemical equation that shows only the ions that participate in the reaction. It is useful for understanding the essential chemical change that occurs during a reaction, without the interference of spectator ions.
Spectator ions are ions that are present in the solution but do not participate in the reaction. They do not undergo any chemical change and are found on both sides of the balanced chemical equation.
Net Ionic Equation for Sodium Sulfate and Barium Chloride
The balanced chemical equation for the reaction between sodium sulfate and barium chloride is:
Na 2SO 4(aq) + BaCl 2(aq) → BaSO 4(s) + 2 NaCl(aq)
The spectator ions in this reaction are sodium (Na +) and chloride (Cl –). They are present on both sides of the equation and do not participate in the reaction.
The net ionic equation for the reaction is:
Ba 2+(aq) + SO 42-(aq) → BaSO 4(s)
Applications of Net Ionic Equations
Net ionic equations have various applications in chemistry, including:
- Qualitative analysis: Net ionic equations can be used to identify ions in a solution by observing the formation of precipitates or gases.
- Solubility predictions: Net ionic equations can be used to predict the solubility of ionic compounds by considering the formation of precipitates.
- Predicting the products of a reaction: Net ionic equations can be used to predict the products of a reaction by identifying the ions that will react with each other.
- Determining the relative amounts of products: Net ionic equations can be used to determine the relative amounts of products formed in a reaction by considering the stoichiometry of the reaction.
Limitations of Net Ionic Equations
Net ionic equations have some limitations:
- They do not provide information about reaction rates or equilibrium constants.
- They do not consider the presence of weak electrolytes or the effect of pH on the reaction.
Key Questions Answered
What is the net ionic equation for sodium sulfate and barium chloride?
The net ionic equation for the reaction between sodium sulfate and barium chloride is: Na+(aq) + SO42-(aq) + Ba2+(aq) + Cl-(aq) → BaSO4(s) + 2Na+(aq) + 2Cl-(aq)
What are the spectator ions in the reaction between sodium sulfate and barium chloride?
The spectator ions in the reaction between sodium sulfate and barium chloride are sodium ions (Na+) and chloride ions (Cl-).
What are the applications of net ionic equations?
Net ionic equations have various applications in chemistry, including qualitative analysis, solubility predictions, and understanding the stoichiometry of reactions.